Abstract
Introduction Belantamab mafodotin is an antibody drug conjugate targeting B-cell maturation antigen (BCMA) with single agent activity around 32% in the DREAMM2 phase II trial (Lonial et al, Cancer 2021). Side effects, including keratopathy and reduced best corrected visual acuity (BCVA), often necessitate dose reduction and dose delays which may limit efficacy. The aim of this study was to assess response rates, dose modifications, and frequency of ocular adverse events in patients treated with belantamab mafodotin in a real-world setting and in relation to prior treatment with BCMA targeted therapies.
Methods All patients treated with at least one dose of commercial belantamab mafodotin at Memorial Sloan Kettering Cancer Center between October 1, 2020 and July 11, 2022 were included in the study. Descriptive statistics were used to assess patient characteristics, response rates, and rate of adverse events. Kaplan Meier curves were used to assess progression free survival (PFS) and overall survival (OS).
Results Eighty-two relapsed/refractory multiple myeloma patients were included; 40 (49%) were women median age was 68 years, and 50 patients (61%) had high risk cytogenetics. Twelve patients were treated on the Expanded Access Program with belantamab mafodotin prior to receiving commercial drug. Patients had a median of 6 (range 2-14) prior lines of therapy, all had received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody. Sixteen patients (20%) had received one or more BCMA-targeted agents including a bi-specific antibody (N=5), chimeric antigen receptor (CAR) T cell (N=12), and trial therapy with belantamab mafodotin (N=1).
Patients received a median of 4 cycles (range 1-31) of belantamab mafodotin. The majority, 96% (N=79), as single agent, while 3 patients were received belantamab mafodotin in combination with other anti-myeloma agents.
The overall response rate (ORR) in all patients was 45%; 37/82 patients. Of these, 10 achieved a partial response (PR); 14 patients achieved a very good partial response (VGPR), and 13 patients achieved a complete response (CR) (Table). The median duration of response for patients in the ORR cohort was 11 months. Eighteen patients had stable disease (SD) and 27 patients had progressive disease (PD). The median PFS was 6 months and median OS was not reached. At the end of follow up, 19 patients continued belantamab mafodotin therapy and 28 patients had died. In a separate analysis of patients not included in the Expanded Access Program, the ORR was 43%; 8 had a PR, 13 had a VGPR, and 8 patients had a CR.
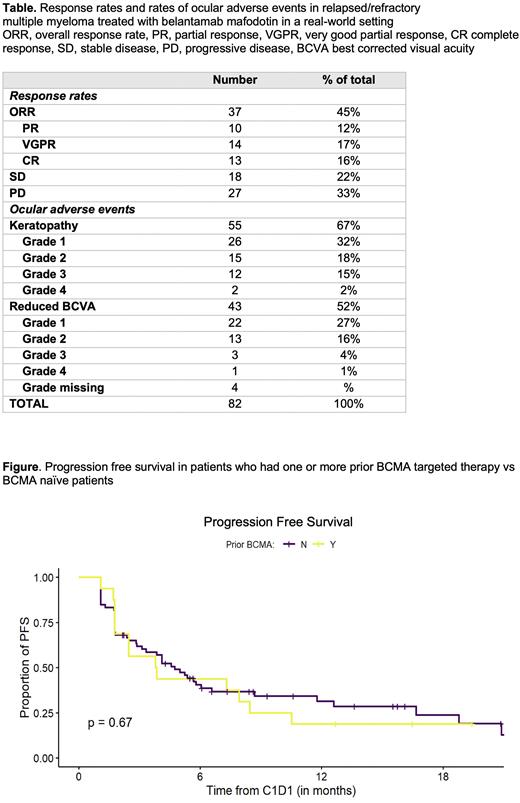
Of the 16 patients who had prior exposure to BCMA targeted therapy, 5 patients had a PR or better (3 VGPR, 2 CR), 2 patients were in a VGPR/CR at start and continued to respond, 3 patients had SD while 6 patients had PD. The median PFS was similar in patients that were BCMA exposed and those that were BCMA naïve (Figure).
Fifty-six patients (68%) had any grade of ocular toxicity per the Keratopathy and Visual Acuity (KVA) scale; 26 patients had grade 1, 15 grade 2, 12 grade 3, and 2 patients had grade 4 keratopathy, respectively. Twenty-six patients had presence of corneal microcysts (information missing in 11 patients). Forty-five patients experienced a decline in BCVA; 22 patients had grade 1, 13 had grade 2, 3 had grade 3, and 1 patient had grade 4 decline in BCVA, respectively (grade missing in 4 patients).
The dose of belantamab mafodotin was reduced from 2.5 mg/kg to 1.92 mg/kg in 36 patients while 29 patients had dose delays, the majority due to ocular adverse events but also due to baseline cytopenia, infection, and patient preference. Most patients who had dose modifications due to ocular events were able to continue therapy on a lower dose with maintained response. Nine patients discontinued treatment due to keratopathy, 3 of whom had ongoing grade 1 keratopathy at the end of follow up.
Conclusion In this heavily pre-treated multiple myeloma patient population, the ORR was 43-45% which is similar or better than the DREAMM2 phase II trial. The rate of ocular toxicity (68% keratopathy) was comparable to previous reports. Importantly, 5 of 16 patients with exposure to prior BCMA exposure achieved a PR or better while 5 had SD. The PFS was similar in BCMA exposed and BCMA naïve patients. These are encouraging outcomes for belantamab mafodotin in the real-world setting with responses and toxicities comparable to those reported in clinical trials.
Disclosures
Hultcrantz:Amgen, Daichii Sankyo, Cosette, GSK: Research Funding; Curio Science LLC: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Intellisphere LLC: Consultancy. Hassoun:Janssen Pharmaceuticals: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Korde:Amgen, Janssen: Research Funding; Clinical Care Options, OncLive, Intellisphere: Consultancy. Mailankody:Juno Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Fate Therapeutics: Research Funding; Plexus Communication: Honoraria; OncLive: Honoraria; Physician Education Resource: Honoraria; Memorial Sloan Kettering Cancer Center: Current Employment; Legend Biotech: Consultancy; Evicore: Consultancy; Janssen Oncology: Consultancy, Research Funding; BioAscend: Consultancy; Optum Oncology: Consultancy; Allogene Therapeutics: Research Funding; Takeda Oncology: Research Funding. Shah:MashUpMD: Honoraria; ACCC: Honoraria; MJH Lifesciences: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Sanofi: Consultancy. Tan:Janssen: Consultancy, Research Funding. Lahoud:MorphoSys, Inc: Membership on an entity's Board of Directors or advisory committees. Landau:Celgene: Consultancy; Prothena: Honoraria; Pfizer: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Karyopharm: Consultancy; Juno: Consultancy; Caelum Biosciences: Consultancy; Legend Biotech USA Inc: Consultancy; Takeda Pharmaceuticals: Consultancy, Other: grants/pending grants; Janssen: Consultancy; Alexion: Other: grants/pending grants; Janssen Scientific Affairs, LLC: Other: grants/pending grants. Scordo:Angiocrine Bioscience, Inc.: Consultancy, Research Funding; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); McKinsey & Company: Consultancy; i3Health (CME): Honoraria; Medscape, LCC (CME): Honoraria. Shah:Janssen: Research Funding; Amgen: Research Funding; Beyond Spring: Research Funding. Giralt:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Actinuum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; MILTENYI: Research Funding; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Research Funding; OMEROS: Research Funding; SPECTRUM Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Lesokhin:Pfizer, Genmab, Sanofi, Iteos, BMS, Janssen: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Janssen, Pfizer, BMS, Genentech/Roche: Research Funding; Janssen, Pfizer, Iteos, Sanofi, Genmab: Honoraria; Serametrix, inc: Patents & Royalties; Trillium Therapeutics: Consultancy, Research Funding; Sanofi: Research Funding; BMS: Honoraria; Amgen: Honoraria. Usmani:Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding; Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal